Which Statement Best Describes the Electronegativity of an Element
Science Chemistry QA Library Which one of the following statements best describes electronegativity in atoms. Which statement best.

Solved Which One Of The Following Statements Best Describes Electronegativity In Atoms A Electronegativity Is What Happens When An Atom Gains An Electron To Become An Anion B Electronegativity Is The Attraction An
Electronegativity tends to be higher in the metals than in the nonmetals.

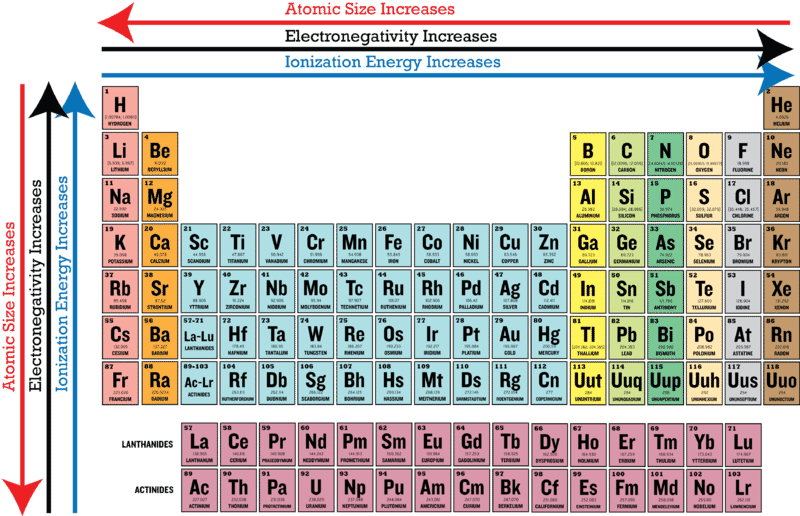
. Electronegativity decreases with an increasing atomic number within a period. B Electronegativity of an atom is its ability to attract electrons during bond formation. Which statement accurately describes a pattern in the size of atomic radii in the Periodic Table of the Elements.
Which statement is true about SO42. Find step-by-step Chemistry solutions and your answer to the following textbook question. B Electronegativity is the attraction an elements nucleus has for the electrons in a chemical bond.
B Each successive element has a lower electronegativity C All elements have similar chemical properties. Element A has an electronegativity of 08 and element B has an electronegativity of 30. Which term best describes C2O42.
O The compound is largely ionic with X as the anion. Which statement best describes the name and location on the Periodic Table of the elements with the highest and lowest electronegativity respectively. B Electronegativity of an atom is its ability to attract electrons during bond formation.
8 and element b has an electronegativity of 1. A Each successive element has a greater atomic radius. Which statement best describes the bonding in a-b.
The ability of an atom to lose electrons C. Values increase from left to right across a period and decrease down a group. Which statement describes the trends of electronegativity values in the periodic table.
Which statement best describes the bonding in A3B. A Electronegativity is what happens when an atom gains an electron to become an anion. It is a polyatomic anion because it has more than one atom and a negative charge.
On A The compound is ionic with A is the cation Moving to. For the hypothetical compound AX2 which statement is true. A The AB bond is largely covalent with a δ- on A.
Which statement does BEST describe the electronegativity values of the elements shown. Element a has an electronegativity of 2. Junior High School.
Chemistry questions and answers. Element A has an electronegativity of 08 and element B has an electronegativity of 30. Which statement best describes the bonding in X3Y.
Element x is belong to group 1which of the following best describes element x. Which statement best describes the electronegativity of an element. The AB bond is polar covalent with a on A The compound is ionic with A is the anion The AB bond is polar covalent with a 8.
See answer 1 Best Answer. Asked Sep 16 2016 in Chemistry by Ademplet. A Electronegativity of an atom is its ability to produce energy while losing an electron.
Which of the following statements below describes electronegativity. How good an atom in attracting electrons B. Electronegativity decreases as you move down in a column.
Electronegativity is a chemical property that describes the tendency of an atom to attract a shared pair of electrons or electron density towards itself. Which statement best describes the electronegativity of an element. The energy required to remove an electron from a specific atom D.
The XY bond is largely covalent with a S on X. Electronegativity is a chemical property that describes the tendency of an atom to attract a shared pair of electrons or electron density towards itself. Which statement best describes the bonding in A3B.
Answer choices Helium on the upper right has the highest electronegativity and Francium on the lower left has the lowest electronegativity. Values increase from left to right across a period and increase down a group. Electronegativity tends to be lower in the gases than in the solids.
Which statement describes the elements in Period 3. Hypothetical element X has an electronegativity of 08 and hypothetical element Y has an electronegativity of 30. B Electronegativity of an atom is its ability to attract electrons during bond formation.
Answer choices Atomic radii decrease from left to right across a period and decrease from top to bottom in a group. Your email address will not be published. Leave a Reply Cancel reply.
The ionization energies kJmol of hydrogen H nitrogen N. A is a metal from Group 2A and X is a nonmetal from Group 7A. Values decrease from left to right across a period and increase down a group.
Required fields are marked.

Electronegativity Basic Introduction Periodic Trends Which Element Is More Electronegative Youtube

No comments for "Which Statement Best Describes the Electronegativity of an Element"
Post a Comment